A Carbon Atom Can Form Up To Four Covalent Bonds - Carbon can form four covalent bonds to create an organic molecule. Carbon can form four covalent bonds to create an organic molecule. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Well, carbon can form up to four covalent bonds. A carbon atom has six electrons in its. A carbon atom can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form.
The _____ functional group(s) is/are found within amino acids, while carbohydrates. Well, carbon can form up to four covalent bonds. Carbon can form four covalent bonds to create an organic molecule. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon has four valence electrons, so it can achieve a full outer energy level by. A carbon atom can form up to four covalent bonds. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its.
Carbon has four valence electrons, so it can achieve a full outer energy level by. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its. Well, carbon can form up to four covalent bonds. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon can form four covalent bonds to create an organic molecule. A carbon atom can form up to four covalent bonds.
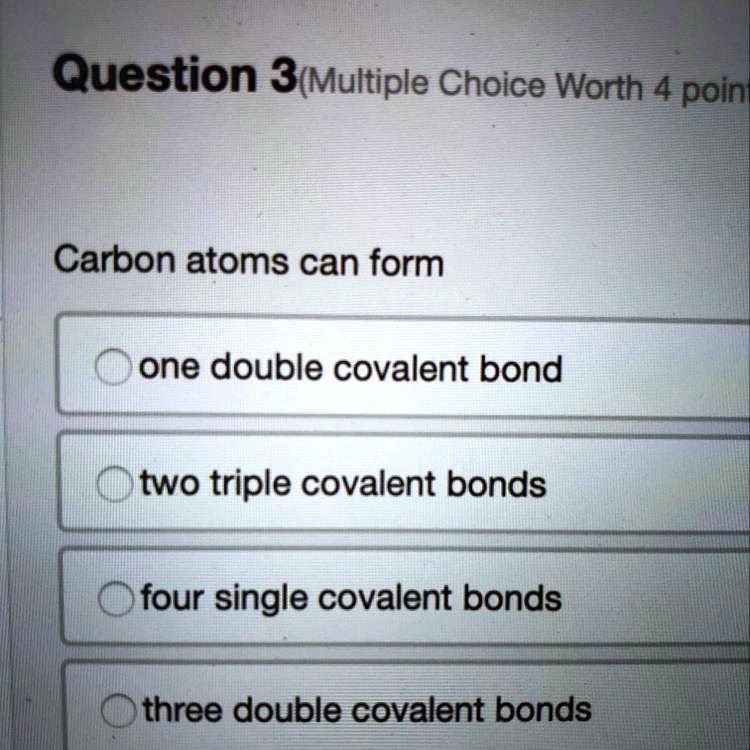
SOLVED Carbon atoms can form... A) one double covalent bond B) two
The _____ functional group(s) is/are found within amino acids, while carbohydrates. A carbon atom can form up to four covalent bonds. Well, carbon can form up to four covalent bonds. A carbon atom has six electrons in its. Carbon has four valence electrons, so it can achieve a full outer energy level by.
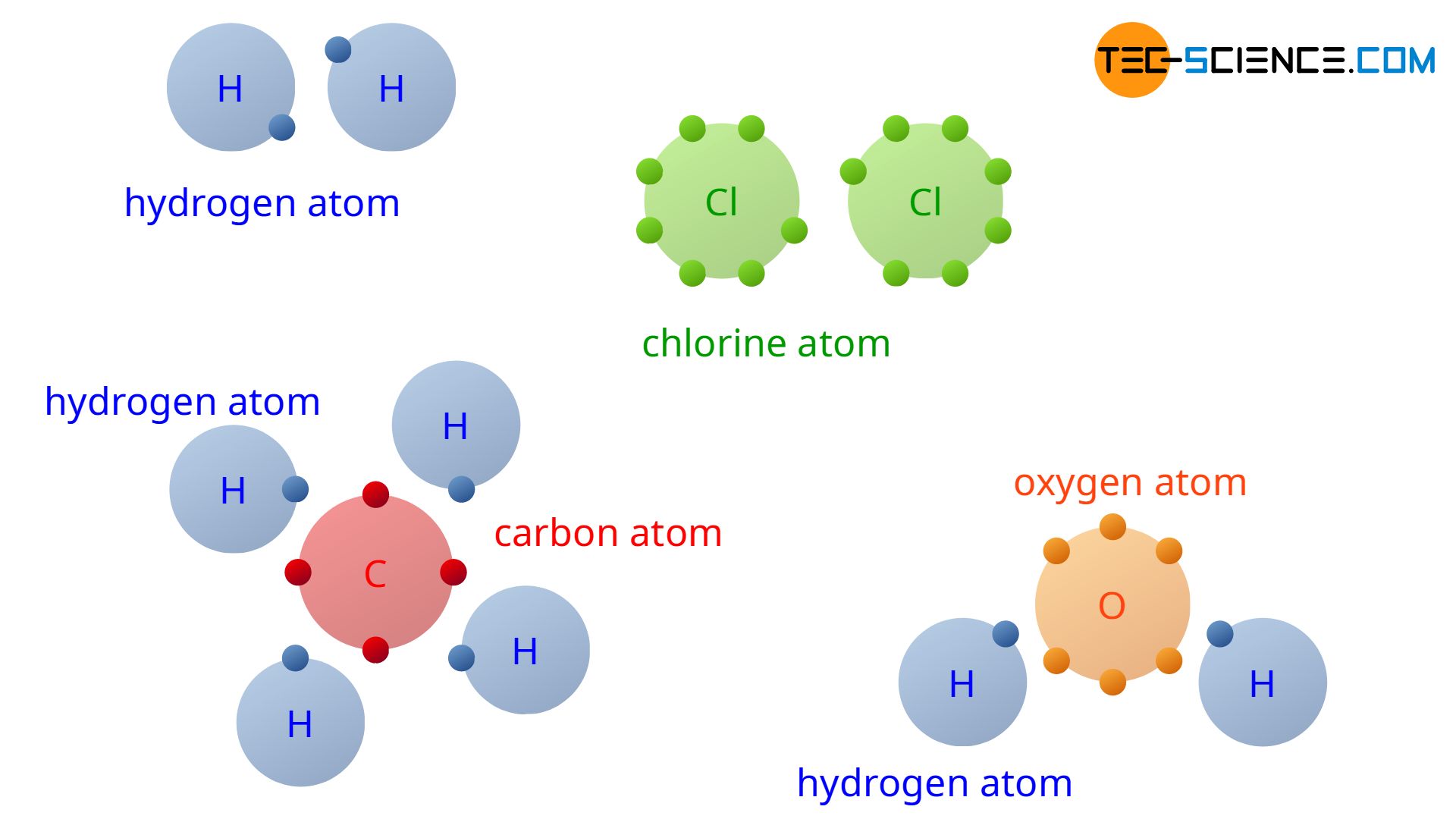
Covalent bonding tecscience
A carbon atom has six electrons in its. Well, carbon can form up to four covalent bonds. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon has four valence electrons, so it can achieve a full outer energy level by. A carbon atom can form up to four covalent bonds.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The _____ functional group(s) is/are found within amino acids, while carbohydrates. A carbon atom has six electrons in its. Well, carbon can form up to four covalent bonds. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. A carbon atom can form up to four covalent bonds.
CH104 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Carbon can form four covalent bonds to create an organic molecule. Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon can form four covalent bonds to create an.
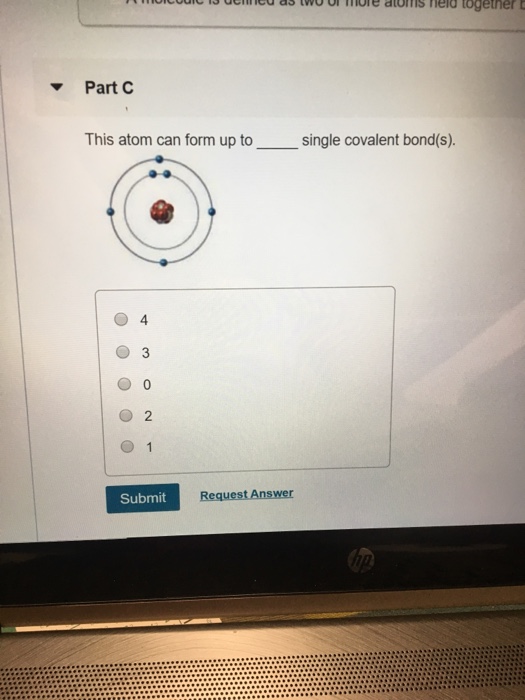
Solved Part C This atom can form up to single covalent
Carbon can form four covalent bonds to create an organic molecule. Carbon can form four covalent bonds to create an organic molecule. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Well, carbon can form up to four covalent bonds. A carbon atom has six electrons in its.
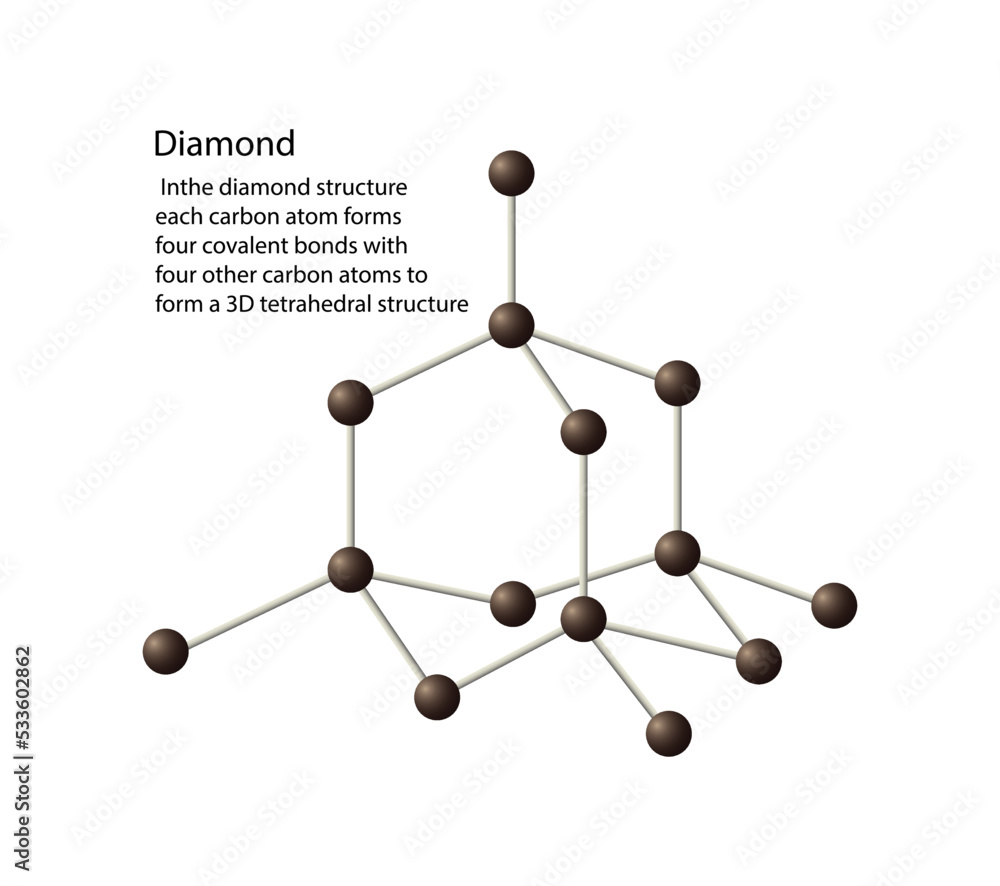
Vetor de illustration of chemistry, The diamond structure each carbon
The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon has four valence electrons, so it can achieve a full outer energy level by. A carbon atom can form up to four covalent bonds. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Well, carbon can form up to four covalent bonds.
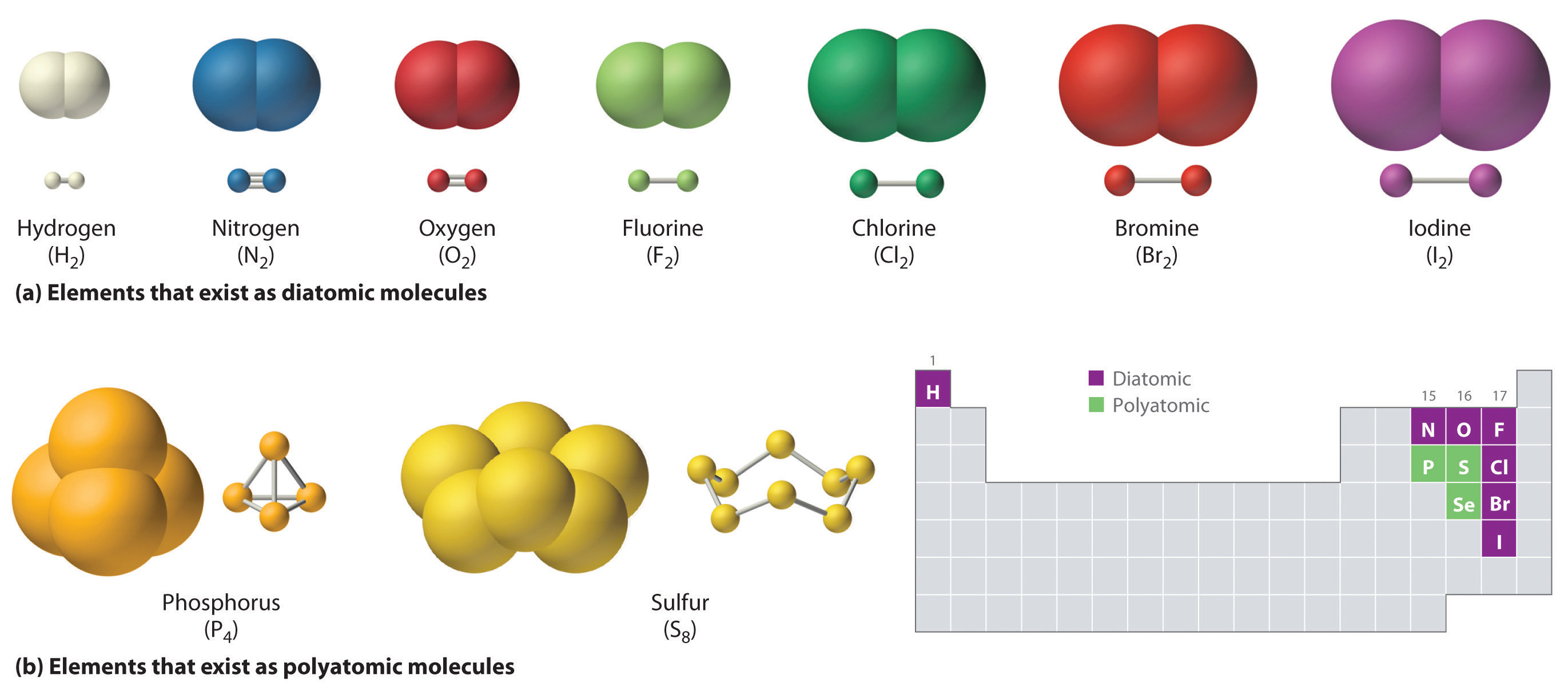
__TOP__ How Many Covalent Bonds Can Chlorine Form
Carbon can form four covalent bonds to create an organic molecule. Carbon has four valence electrons, so it can achieve a full outer energy level by. The _____ functional group(s) is/are found within amino acids, while carbohydrates. A carbon atom has six electrons in its. Study with quizlet and memorize flashcards containing terms like a carbon atom can form.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
Carbon can form four covalent bonds to create an organic molecule. A carbon atom can form up to four covalent bonds. A carbon atom has six electrons in its. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon can form four covalent bonds to create an organic molecule.
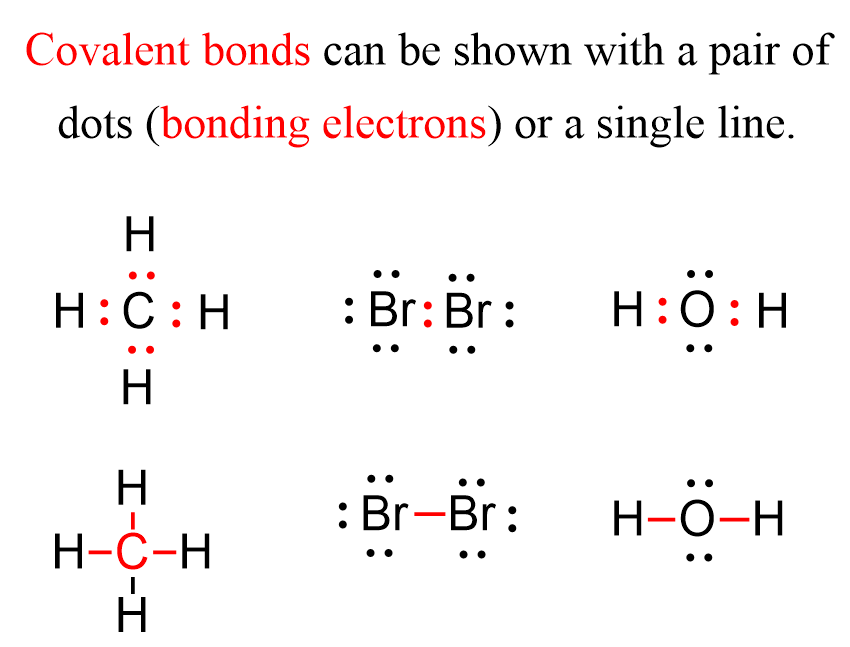
Covalent Bond Chemistry Steps
A carbon atom can form up to four covalent bonds. A carbon atom has six electrons in its. Carbon can form four covalent bonds to create an organic molecule. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. The _____ functional group(s) is/are found within amino acids, while carbohydrates.
SOLVED Mark the following statements about carbon as true or false A
The _____ functional group(s) is/are found within amino acids, while carbohydrates. Well, carbon can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon can form four covalent bonds to create an organic molecule.
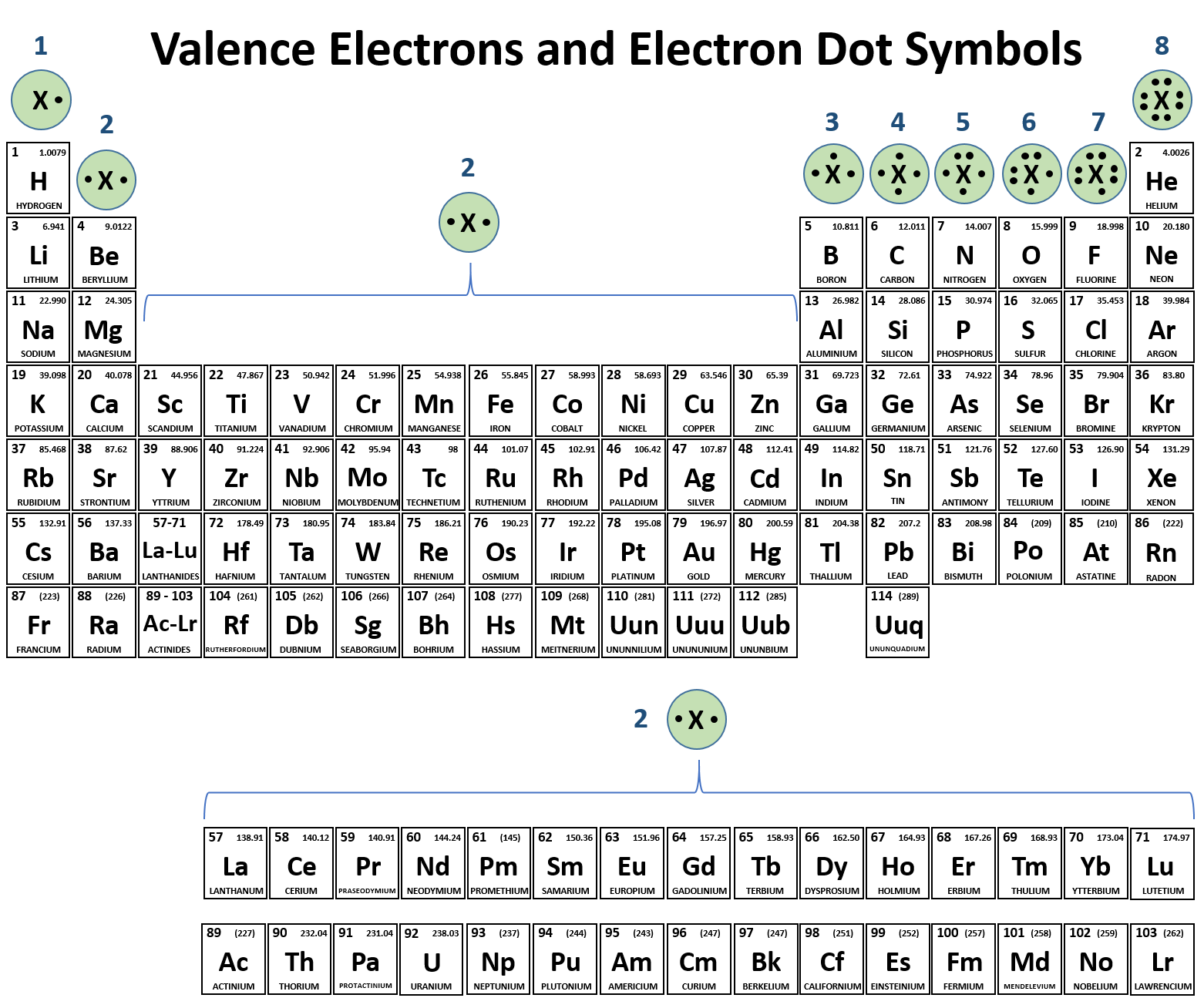
Carbon Has Four Valence Electrons, So It Can Achieve A Full Outer Energy Level By.
Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Well, carbon can form up to four covalent bonds. A carbon atom can form up to four covalent bonds. Carbon can form four covalent bonds to create an organic molecule.
The _____ Functional Group(S) Is/Are Found Within Amino Acids, While Carbohydrates.
A carbon atom has six electrons in its. Carbon can form four covalent bonds to create an organic molecule.