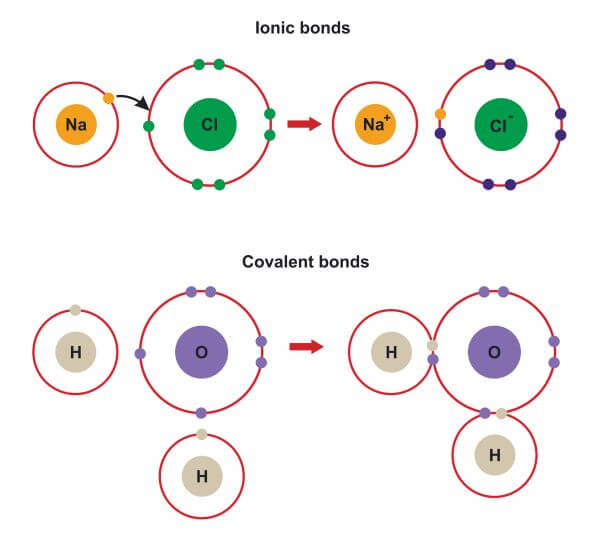
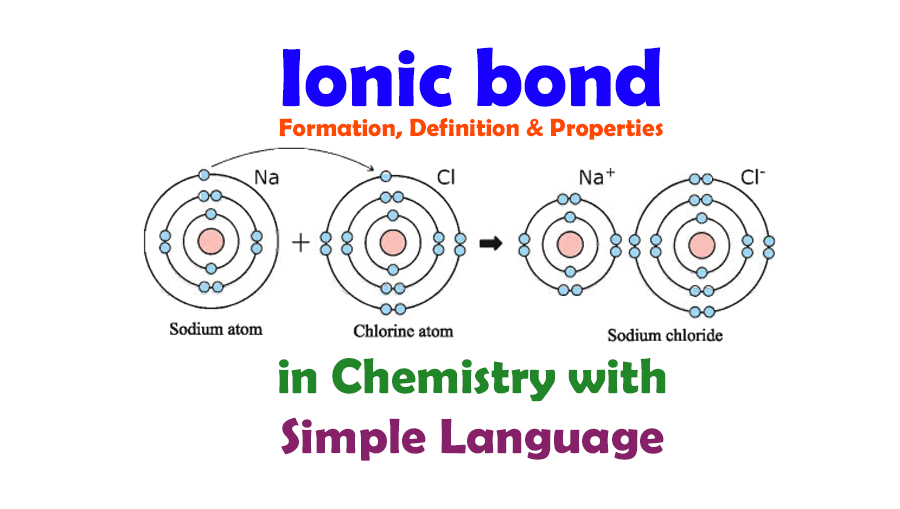
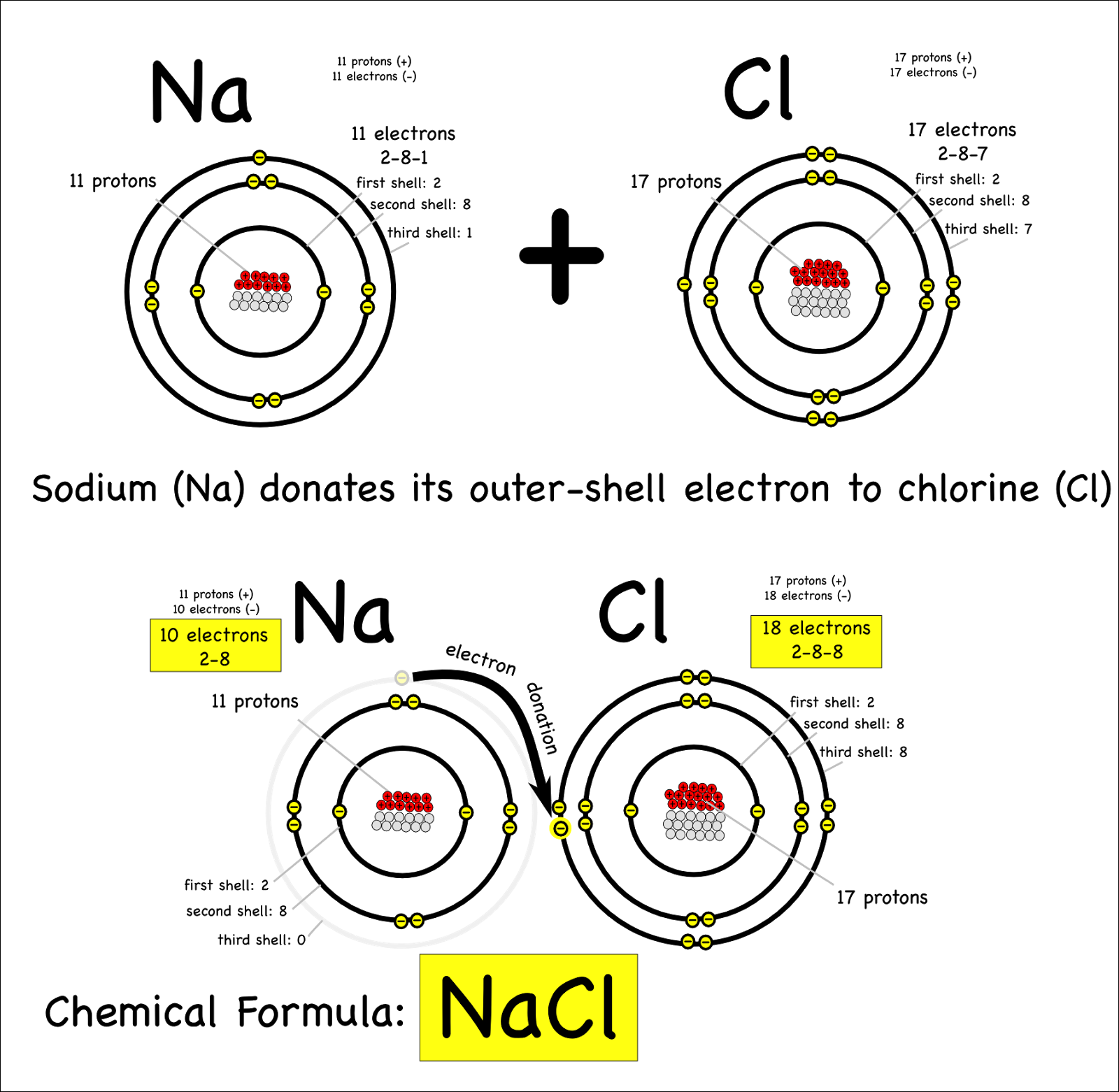
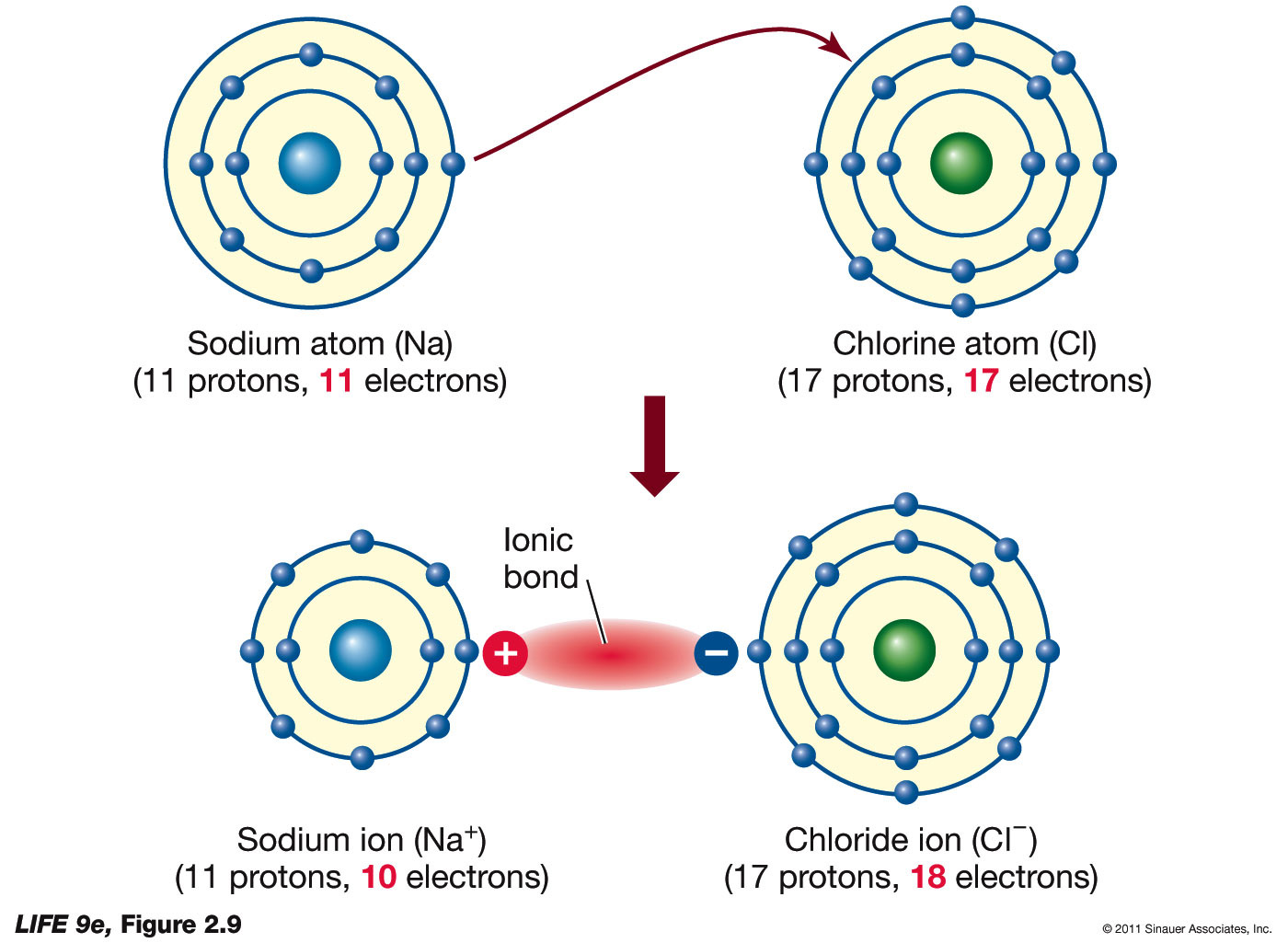
An Ionic Bond Forms Between A - A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms.
A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations and anions.
In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion.
Ionic Bond Examples Biology Dictionary
A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
Ionic Bond and Ionic Bond Formation, Definition, Properties in
Ionic bonds are formed between cations and anions. A cation is formed when a metal ion. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonding is the complete transfer of valence electron(s).
Diagram Of Ionic Bonding
Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s).
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
Ionic Bonding Diagram
In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
Examples of Ionic Bonds and Compounds
Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. In an ionic bond, a complete transfer of electrons takes place in the process of bond. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
Ionic Bond Facts, Definition, Properties, Examples,, 55 OFF
Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations.
ionic bond Definition, Properties, Examples, & Facts Britannica
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations.
Diagram Ionic Bond
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process.
An Ionic Bond Forms Between A ____ Ion With A Positive Charge And A ____ Ion With A Negative.
Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s) between atoms.




.PNG)